| Journal of Clinical Gynecology and Obstetrics, ISSN 1927-1271 print, 1927-128X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Gynecol Obstet and Elmer Press Inc |
| Journal website https://www.jcgo.org |
Original Article
Volume 12, Number 3, December 2023, pages 71-77
An Australian and Aotearoa New Zealand Audit of Obstetric Fluid Management During Induction of Labor
Darren J. Lowena, b, f , Paul Howatc
, Russell Hodgsond, e
aDepartment of Anaesthesia & Perioperative Medicine, Northern Health, Epping, Victoria, Australia
bDepartment of Critical Care, Melbourne Medical School, The University of Melbourne, Parkville, Victoria, Australia
cDepartment of Obstetrics & Gynaecology, Northern Health, Epping, Victoria, Australia
dDivision of Surgery, Northern Health, Epping, Victoria, Australia
eDepartment of Surgery, University of Melbourne, Parkville, Victoria, Australia
fCorresponding Author: Darren J. Lowen, Department of Anaesthesia & Perioperative Medicine, Northern Health, Epping, Victoria 3076, Australia
Manuscript submitted October 19, 2023, accepted December 7, 2023, published online December 28, 2023
Short title: Obstetric Fluid Management During Induction of Labor
doi: https://doi.org/10.14740/jcgo924
| Abstract | ▴Top |
Background: Although guidelines are in place for obstetric indications that warrant an induction of labor, no such guidelines or policies exist for the management of fluids during labor. This extends to the concentration of oxytocin used for induction, maintenance rate of intravenous crystalloids and subsequent boluses of this crystalloid. The aim of this survey was to obtain a snapshot of current Australian and Aotearoa New Zealand obstetric practice as it pertains to fluid management during induction of labor.
Methods: A REDCap survey was made available to medical practitioners registered with the Australian and New Zealand College of Obstetricians and Gynecologists (RANZCOG) via email.
Results: A total of 287/6,707 (4.3%) medical practitioners registered with RANZCOG completed the survey. A response was received from medical practitioners in all states and territories of Australia and Aotearoa New Zealand, from a total of 140 hospitals. The majority of respondents were consultants (178/287 (62%)). Variability was noted for the concentration of oxytocin that was used. Three different types of crystalloid were used. Marked variability was noted for the rate of infusion of crystalloid, the indications for a bolus of crystalloid and the size of this bolus.
Conclusions: The management of fluid during induction of labor has marked variability both in Australia and Aotearoa New Zealand, suggesting that a policy to guide this management does not exist, and best practice has not been identified.
Keywords: Parturition; Oxytocin; Crystalloid solution; Induction; Labor
| Introduction | ▴Top |
There has been a steady increase in the requirement for an induction of labor [1]. Despite evidence demonstrating that fluid management is critical in the perioperative setting, there is minimal evidence to guide treatment, or policies pertaining to the management of intravenous fluids during induction of labor [2, 3]. It is acknowledged that certain predisposing medical conditions, for example, pre-eclampsia, cardiac disease, allow for a restricted fluid regime.
Prolonged labor is known to be associated with a higher instrumental delivery rate and emergency cesarean section rate, both of which are more closely associated with postpartum hemorrhage [4]. There is some evidence that large fluid boluses may decrease uterine contraction, although this is contraindicated by other evidence supporting a high continuous rate of intravenous fluids [5, 6]. There remains equipoise in the literature regarding the quantity of fluid required during induction of labor, due to the low numbers, differing types and rates of fluid and differing requirements for women to remain nil per os [7].
Intravenous fluid administration intrapartum has been associated with postpartum consequences, such as fuller and firmer breasts, which is more likely to be edema rather than engorgement, suggesting that this may contribute to difficulty with early breast feeding [8]. Birth weight and delayed lactogenesis have also been linked to intravenous fluid delivery during labor [9].
With no clear evidence during induction of labor to support either a certain continuous rate of intravenous fluid or fluid boluses, the aim was to assess the current practice of practitioners registered with Royal Australian and New Zealand College of Obstetricians and Gynecologists (RANZCOG), in relation to intravenous fluid management during induction of labor. This may inform on further studies, to determine if there is a correlation between the use of intravenous fluids and maternal and/or neonatal morbidity.
| Materials and Methods | ▴Top |
In this cross-sectional study, medical practitioners registered with RANZCOG were invited to participate in an anonymous electronic questionnaire, which was emailed to the address registered with RANZCOG. As per the 2021 Activities Report published by RANZCOG and available electronically, medical practitioners include the following: FRANZCOG trainees 699 (10.4%), fellows 2,378 (35.5%), diplomates 2,536 (37.8%) and CWH/DRANZCOG trainees 1,094 (16.3%). A reminder email to participate was emailed 2 weeks after the initial email. Study data were collected and managed using REDCap [10, 11]. A total of 11 statements required a response, with branched logic used if further information was required, relative to the response. Scenarios possibly requiring a bolus of intravenous fluids were taken from direct observation of fluid bolus use by the primary author. Consent was implied by the final submission of responses. The first response was obtained on November 12, 2021, and the last response to the survey was obtained on December 13, 2021. Survey results were reported using descriptive analysis only. This study was approved by the Northern Health Research Office (HREC/74856/NH-2021-259004) on May 7, 2021, and by RANZCOG. All reported research was conducted in accordance with the principles set forth in the Helsinki Declaration 2008.
| Results | ▴Top |
Two hundred eighty-seven medical practitioners registered with RANZCOG responded out of a total of 6,707 (4.3%). Unfortunately, within the time frame allowed, 6,420 (95.7%) medical practitioners did not respond. Two hundred sixty-six respondents were from Australia and 21 respondents listed Aotearoa New Zealand as their primary residence. All states and territories of Australia were represented. Victoria had the greatest number of respondents, and most respondents listed their primary place of work as being a public institution (216 respondents (75.3%)) (Fig. 1). Most of the respondents were consultant obstetricians (178 (62%)). Three different types of crystalloid were used, these being Hartmann’s solution, sodium chloride 0.9% solution, or Plasma-Lyte 148, with Hartmann’s solution being the preferred crystalloid used as indicated by 68.3% of the respondents. The next preferred crystalloid was sodium chloride 0.9% at 27.9%. Plasma-Lyte 148 was used by 3.1% of the respondents and there were two blank responses to this question.
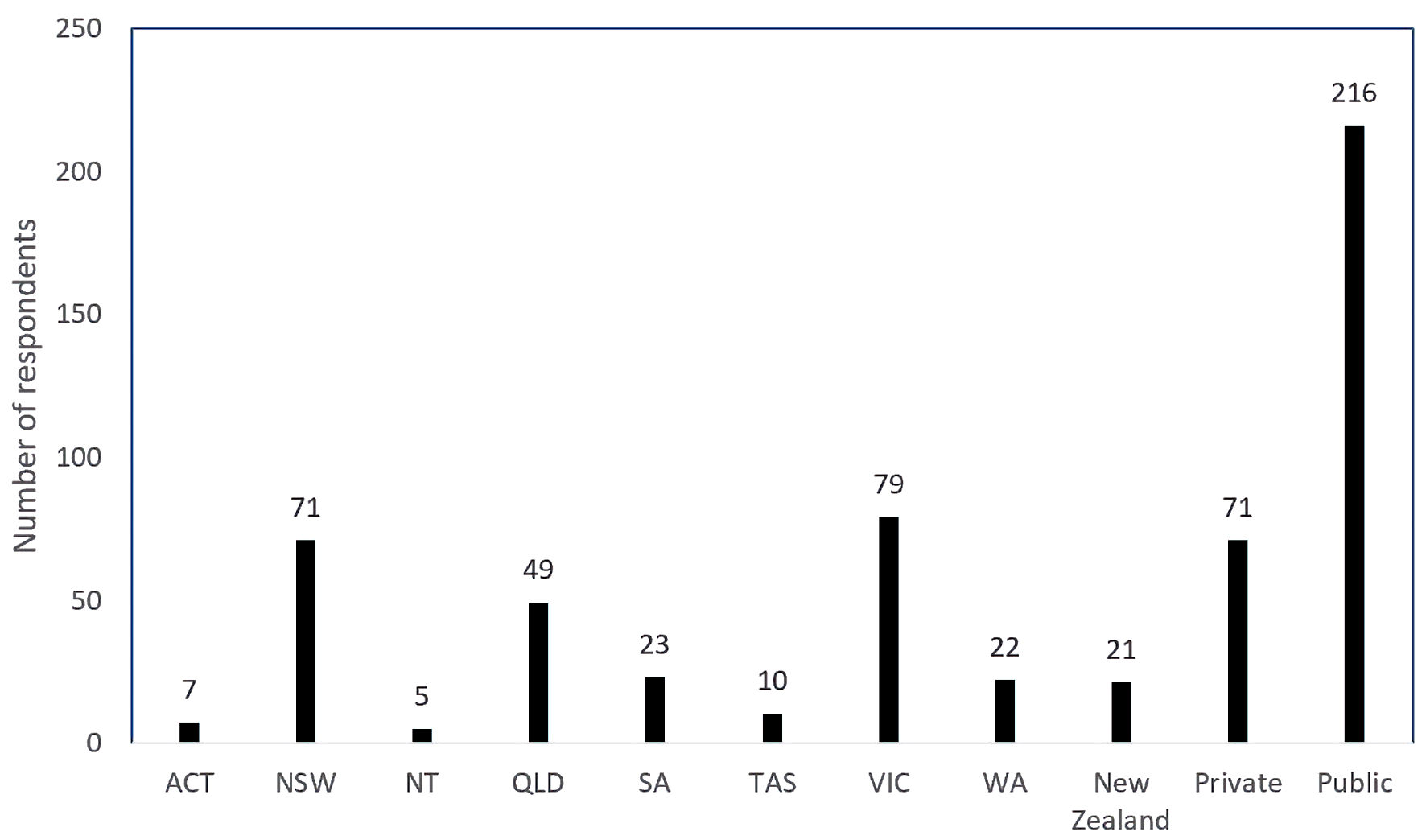 Click for large image | Figure 1. Number of respondents per state (of Australia) and New Zealand and private versus public. ACT: Australian Capital Territory; NSW: New South Wales; NT: Northern Territory; QLD: Queensland; SA: South Australia; TAS: Tasmania; VIC: Victoria; WA: Western Australia. |
Concentration of oxytocin used
The most frequent concentration of oxytocin used was 10 IU in 1,000 mL crystalloid (57.5%). A total of 11 different concentrations of oxytocin were listed (Fig. 2). All states and territories listed more than one concentration of oxytocin being used. More than one concentration of oxytocin was also listed by Aotearoa New Zealand respondents.
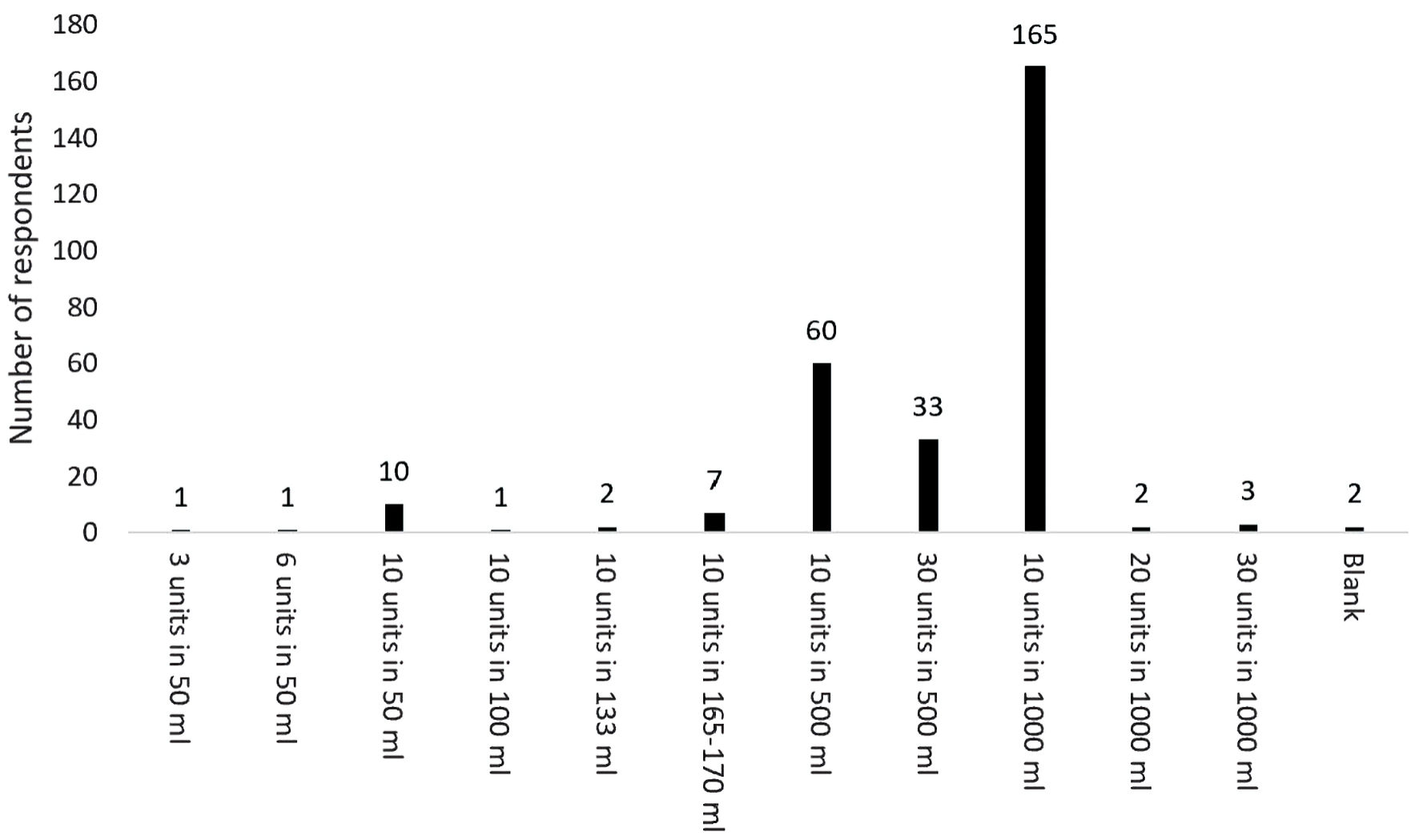 Click for large image | Figure 2. Variability in concentration of oxytocin used for induction of labor. |
Is an accessory intravenous line for crystalloid used?
Most of the respondents indicated that an accessory intravenous fluid infusion was used (226 respondents (78.7%)). For those respondents who listed a single value, the rate with the greatest frequency was 125 mL/h with 92 respondents listing this rate. The rate of infusion varied from 40 mL/h to 250 mL/h, and a number of respondents listed a variable rate of infusion (Fig. 3).
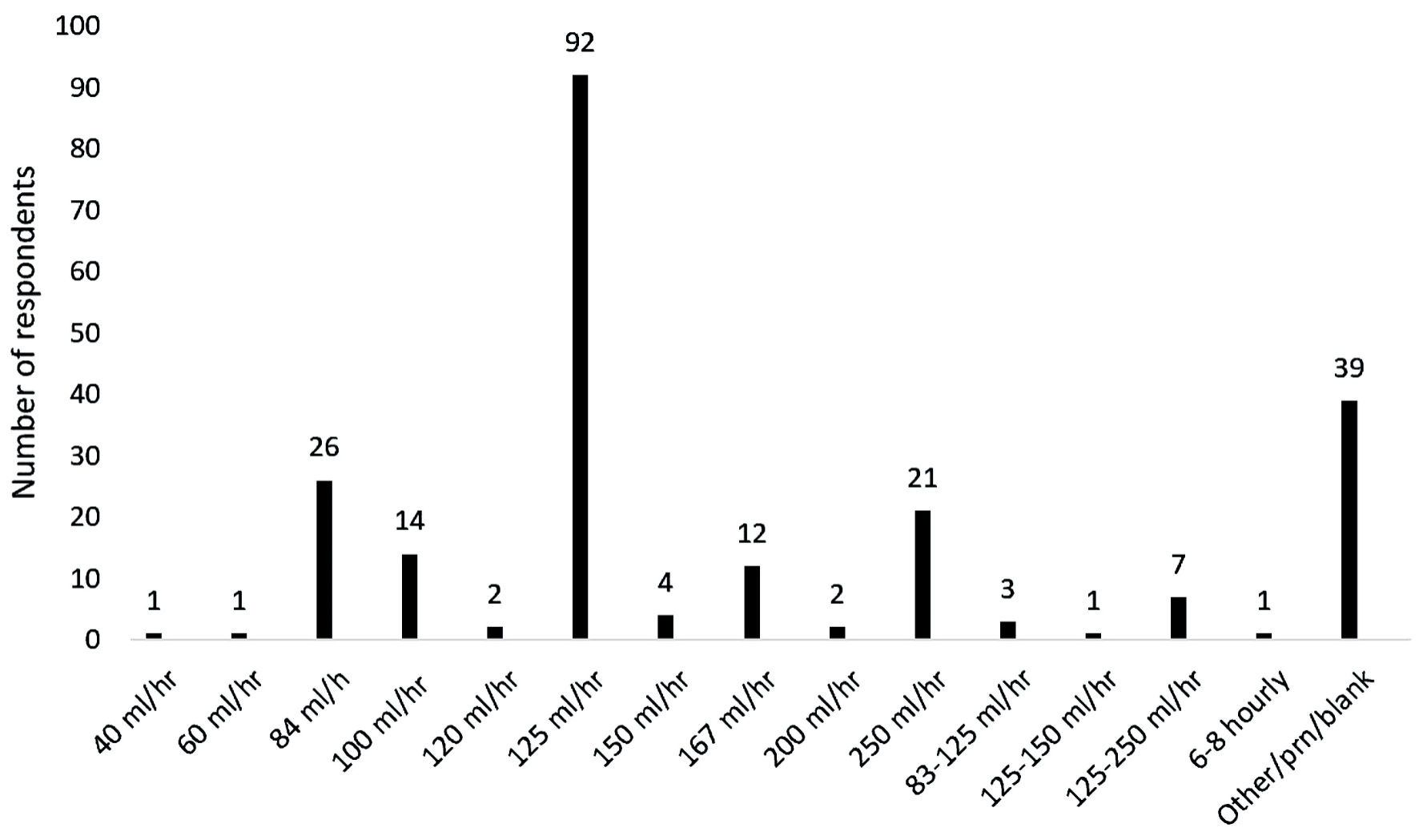 Click for large image | Figure 3. Variability in the rate of background infusion of crystalloid infusion. |
If you believe that the urine is concentrated, or that the amount voided is inadequate, do you request a bolus of fluid?
Most of the respondents (72.1%), encouraged the parturient to void regularly during induction of labor, with 125 (66.8%) giving an intravenous bolus of fluid if the urine was concentrated, or the amount voided was inadequate. A 500 mL bolus volume of intravenous fluid was the most frequent volume, however wide variability with bolus volume was noted (Fig. 4a).
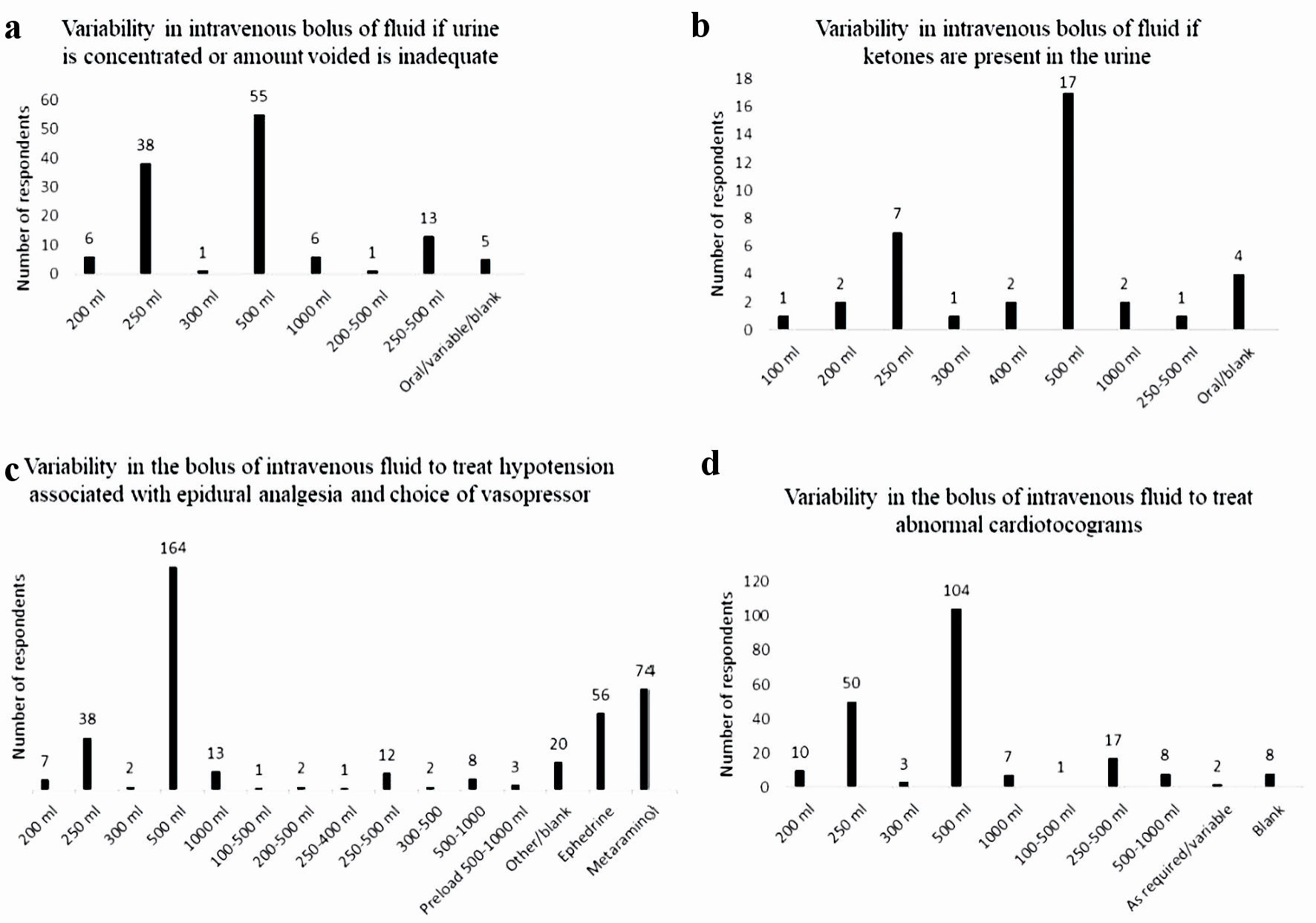 Click for large image | Figure 4. Variability in intravenous fluid boluses for various indication. (a) Variability in intravenous bolus of fluid if urine is concentrated or amount voided is inadequate. (b) Variability in intravenous bolus of fluid if ketones are present in the urine. (c) Variability in intravenous bolus of fluid to treat hypotension associated with epidural analgesia and choice of vasopressor. (d) Variability in intravenous bolus of fluid to treat abnormal cardiotocograms. |
Do you check the urine for ketones?
Most of the respondents did not check the urine for ketones (248 (86.4%)). Of the 37 respondents who did (two blank responses), 500 mL was the most frequent bolus volume given, with a wide variability in bolus volumes noted (Fig. 4b).
If the parturient becomes hypotensive secondary to the placement of a lumbar epidural, is this managed with fluids or a vasoconstrictor?
Nearly all of the respondents manage hypotension secondary to the establishment of epidural analgesia with a bolus of fluid (273 respondents (95.1%)). The historical practice of preloading prior to the commencement of epidural still exists, although it was only noted by three respondents. A 500 mL bolus volume was the most frequent bolus volume listed (164 respondents), with the range of bolus volumes being 200 - 1,000 mL. A range of bolus volume rather than a single bolus volume was also noted by 26 respondents (Fig. 4c). Metaraminol was the vasopressor of choice listed by 74 respondents, with 56 respondents listing ephedrine instead. Fourteen respondents listed both metaraminol and ephedrine as the vasopressors used to treat hypotension secondary to the commencement of epidural analgesia (Fig. 4c).
If the cardiotocogram (CTG) is non-reassuring, in addition to parturient positional changes, turning off or turning down the rate of intravenous oxytocin, do you request a bolus of fluid?
Most of the respondents treated a non-reassuring CTG with a bolus of fluid (210 respondents (73.2%)). The most frequent bolus volume was 500 mL (range: 200 - 1,000 mL), with 28 respondents providing a bolus range, rather than actual volume (Fig. 4d).
Do you actively encourage the parturient to drink fluids during labor?
The majority of respondents (94.4%) encouraged parturients to drink fluids during labor, although to differing extent with occasionally (147 respondents), often (82 respondents) and always (41 respondents) being the frequencies recorded.
| Discussion | ▴Top |
Whilst acknowledging the poor response rate to this survey and the limitations upon analysis that this ensues, we have demonstrated that wide variation in practice exists with regards to management of intravenous fluids during induction of labor. The literature to guide intravenous fluid delivery during induction of labor is limited. There is increasing evidence that fluid balance is very important in surgical patients, with regimens previously seen as restrictive shown to enhance organ preservation and function [2]. Whilst women in labor are not usually as comorbid, or have the underlying pathology of these patients, outcomes such as instrumental delivery, increased cesarean section rate and post-partum outcomes including postpartum hemorrhage, altered birth weight and difficulty in feeding have all been causally linked to intravenous fluids [4, 6, 8, 9]. This study demonstrated marked variability in both continuous infusion of fluids and use of fluid boluses, indicating uncertainty about best practice, thus further research into intravenous fluid management during induction of labor is warranted. This is further reinforced by a recent scoping review of the available evidence for intrapartum maternal hydration and assessment. Despite an extensive review identifying 12 guidelines, none of these guidelines, including six specific ones for induction of labor, were evidence-based guidelines specific to hydration and assessment [12].
There are two main sources of continuous intravenous fluids given to parturients undergoing induction of labor, this being the crystalloid that oxytocin is added to and a maintenance infusion of intravenous crystalloid. Whilst most states and territories in Australia produce state guidelines for induction of labor, the concentration of oxytocin, rate of delivery, requirement for obstetric review to continue with oxytocin beyond a certain rate and the maximum dose allowed differ. For most states and territories, the maximum dose is 32 mU/min, however, for Western Australia the maximum dose is 36 mU/min. New South Wales has the highest dose of oxytocin at 40 mU/min [3, 13-17]. Given that medical staff may move between different health practices, in particular, those that are close to state borders, for safety concerns, it would be prudent to continue research to obtain evidence for the best regimen, thus reducing variability in care and harmonizing practice.
Despite the low percentage of obstetricians responding to this survey, which raises the risk of participation bias, it was surprising to identify at least 11 differing concentrations of oxytocin being used for induction of labor. Although the most concentrated solution of oxytocin identified in this survey was 10 IU in 50 mL crystalloid, (as indicated by 10 respondents), the most frequently described concentration was 10 IU in 1,000 mL crystalloid (as indicated by 165 respondents). Some medical conditions such as pre-eclampsia and cardiac failure necessitate the use of fluid restriction, therefore a more concentrated form of oxytocin for induction of labor is warranted. Although there is no current evidence of harm, there is no obvious reason why oxytocin is made up into a low concentration, which then requires a higher volume to be given during induction of labor.
There is limited evidence to guide the use of intravenous fluids during induction of labor, and as conceded by Dawood et al, there is not even consensus as to whether intravenous fluids are even necessary [7]. Continuous intravenous fluids during induction of labor remains a current practice, and the results from this study support this, with three different types of crystalloid identified, with wide variability noted for the rate of infusion. In addition, parturients are often encouraged to drink throughout labor, although there is a split in the available literature, with some countries favoring a strict nil per os approach. Presumably, this is due to concerns with regards to water intoxication or to the requirement of an empty stomach in preparation for the likelihood of an emergency cesarean section, a practice that was first instituted in the late 1940s although its current use in the era of regional anesthesia and/or rapid sequence induction may be debated [18-21]. Thus, the best evidence to support additional continuous intravenous fluid administration, a meta-analysis demonstrating improved outcomes for nulliparous women in spontaneous labor, is predominantly in women being managed with no oral fluids or solids, which confounds the results [6]. Whether this practice also translates to women undergoing induction of labor, is also yet to be established.
Despite the common use of fluid boluses for each of the indications listed in Figure 4, these are likely based on anecdotal evidence and copying of mentor practice during training, as the literary evidence for these indications is unknown or not supporting. Ketosis and subsequent ketonuria are common in labor, yet its significance remains unknown [22]. Whilst it is stated that “the presence or absence of ketonuria can be used to monitor hydration”, a consistent approach to the determination and management of hydration during induction of labor does not exist [12, 21]. Ketosis cannot be treated with an intravenous bolus of crystalloid solution which does not contain glucose. Transient hypotension associated with the establishment of epidural analgesia is due to relaxation of sympathetic tone and the associated vasomotor relaxation, not hypovolemia and is thus best treated with a vasopressor. The Fetal Surveillance Education Program (FSEP), first established in 2004, does not mention that a non-reassuring CTG trace requires management with a fluid bolus (Dr Mark Beaves, head of Quality Assurance Programs RANZCOG (personal communication)). This contrasts with the fourth edition of the intrapartum fetal surveillance clinical guideline, which is produced by RANZCOG. In “Recommendation 8”, it states that the treatment of a non-reassuring CTG trace with intravenous fluid hydration of the mother is a grade A recommendation (body of evidence can be trusted to guide practice) [23]. This discrepancy and the lack of evidence for management of these other “indications” with a fluid bolus, is perhaps behind the wide variety of bolus volume recorded in this study.
There is yet to be conclusive evidence published of harm to either woman or baby with intravenous fluid management during induction of labor, although a few associations have been established. Excess fluid during labor may be contributing to maternal (and fetal) hyponatremia, and fluid overload has also been associated with pulmonary edema [24, 25]. Excess fluid during labor may also be contributing to excess newborn weight loss [25]. Chantry et al (2011) identified that an intravenous fluid rate greater than 200 mL/h was predictive of newborn weight loss exceeding 10% [26], whereas Watson et al, in an exploratory analysis of a randomized controlled trial, suggested that breastfed newborn weight loss was unlikely to be greater than 7%, provided that the total volume of maternal intravenous fluid volume delivered during labor was 2,500 mL or less [25]. Further research is clearly needed to document mother and baby outcomes following management with intravenous fluid.
Conclusions
This study demonstrated marked variability in obstetric practice throughout Australia and Aotearoa New Zealand, in the use of intravenous fluids during induction of labor. This variation may reflect local policies (or lack thereof), a range of education or anecdotal self-learning. The increased or decreased use of intravenous fluids may have unintended consequences; thus, it may be prudent to undertake further research in this area, with the ultimate aim being to establish appropriate national (and international) guidelines and policies for the use of intravenous fluids during induction of labor.
Acknowledgments
The authors would like to sincerely thank all of the medical practitioners registered with the Royal Australian and New Zealand College of Obstetrics and Gynecology who generously provided their time to answer the questions posed within the electronic survey.
Financial Disclosure
None to declare.
Conflict of Interest
The authors have no conflict of interest.
Informed Consent
Informed consent was obtained.
Author Contributions
Darren J. Lowen: design, planning, conduct, data analysis and manuscript writing - original draft. Paul Howat and Russell Hodgson: design, planning, manuscript writing - review and editing.
Data Availability
Any enquiries regarding supporting data availability of this study should be directed to the corresponding author.
| References | ▴Top |
- AIHW. Australian Institute of Health and Welfare. National Core Maternity Indicators. Mothers & babies Canberra. 2020.
- Kendrick JB, Kaye AD, Tong Y, Belani K, Urman RD, Hoffman C, Liu H. Goal-directed fluid therapy in the perioperative setting. J Anaesthesiol Clin Pharmacol. 2019;(Suppl 1):S29-S34.
doi pubmed pmc - Safer Care Victoria Induction of labour. 2018. (updated August 2018). Available from: https://www.bettersafercare.vic.gov.au/clinical-guidance/maternity/induction-of-labour.
- Flood M, McDonald SJ, Pollock W, Cullinane F, Davey MA. Incidence, trends and severity of primary postpartum haemorrhage in Australia: A population-based study using Victorian Perinatal Data Collection data for 764 244 births. Aust N Z J Obstet Gynaecol. 2019;59(2):228-234.
doi pubmed - Cheek TG, Samuels P, Miller F, Tobin M, Gutsche BB. Normal saline i.v. fluid load decreases uterine activity in active labour. Br J Anaesth. 1996;77(5):632-635.
doi pubmed - Ehsanipoor RM, Saccone G, Seligman NS, Pierce-Williams RAM, Ciardulli A, Berghella V. Intravenous fluid rate for reduction of cesarean delivery rate in nulliparous women: a systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2017;96(7):804-811.
doi pubmed - Dawood F, Dowswell T, Quenby S. Intravenous fluids for reducing the duration of labour in low risk nulliparous women. Cochrane Database Syst Rev. 2013;6:CD007715.
doi pubmed - Kujawa-Myles S, Noel-Weiss J, Dunn S, Peterson WE, Cotterman KJ. Maternal intravenous fluids and postpartum breast changes: a pilot observational study. Int Breastfeed J. 2015;10:18.
doi pubmed pmc - Noel-Weiss J, Woodend AK, Peterson WE, Gibb W, Groll DL. An observational study of associations among maternal fluids during parturition, neonatal output, and breastfed newborn weight loss. Int Breastfeed J. 2011;6:9.
doi pubmed pmc - Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381.
doi pubmed pmc - Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O'Neal L, McLeod L, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019;95:103208.
doi pubmed pmc - Kearney L, Craswell A, Dick N, Massey D, Nugent R. Evidence-based guidelines for intrapartum maternal hydration assessment and management: A scoping review. Birth. 2023.
doi pubmed - Guidelines QC. Induction of labour: Queensland Government; 2022. Available from: www.health.qld.gov/qcg.
- Induction and augmentation of labour: Government of South Australia; 2021. Available from: https://www.sahealth.sa.gov.au/wps/wcm/connect/ac7d37804ee4a27985598dd150ce4f37/Induction+and+Augmentation+of+Labour_PPG_V9_0.pdf?MOD=AJPERES&CACHEID=ROOTWORKSPACE-ac7d37804ee4a27985598dd150ce4f37-ocQLhIl.
- Maternity - Oxytocin for the induction of labour at or beyond term: NSW Government; 2011. Available from: www1.heatlh.nsw.gov.au/pds/ActivePDSDocuments/PD2011_075.pdf.
- Canberra Hospital and Health Services. Clinical guideline. Induction of labour: ACT Government; 2018. Available from: www.health.act.gov.au/sites/default/files/2020-09/Induction%20of%20Labour.pdf.
- Induction of labour policy: Government of Western Australia. WA Country Health Service; 2021 (updated April 2021). Available from: www.wacountry.health.wa.gov.au/About-us/Publications/Policies.
- Ophir E, Solt I, Odeh M, Bornstein J. Water intoxication-a dangerous condition in labor and delivery rooms. Obstet Gynecol Surv. 2007;62(11):731-738.
doi pubmed - Szadok P, Bajorek A, Fluder R. Water intoxication in the course of stimulation of labor with oxytocin. Ginekol Pol. 2021;92(7):534-535.
doi pubmed - Toohill J, Soong B, Flenady V. Interventions for ketosis during labour. Cochrane Database Syst Rev. 2008;2008(3):CD004230.
doi pubmed pmc - Committee on Obstetric P. Committee opinion No. 687: approaches to limit intervention during labor and birth. Obstet Gynecol. 2017;129(2):e20-e28.
doi pubmed - Singata M, Tranmer J, Gyte GM. Restricting oral fluid and food intake during labour. Cochrane Database Syst Rev. 2013;2013(8):CD003930.
doi pubmed pmc - Australian R, Obstetricians NZCo, Gynaecologists. Intrapartum fetal surveillance clinical guideline - fourth edition. 2019.
- Tarnow-Mordi WO, Shaw JC, Liu D, Gardner DA, Flynn FV. Iatrogenic hyponatraemia of the newborn due to maternal fluid overload: a prospective study. Br Med J (Clin Res Ed). 1981;283(6292):639-642.
doi pubmed pmc - Watson J, Hodnett E, Armson BA, Davies B, Watt-Watson J. A randomized controlled trial of the effect of intrapartum intravenous fluid management on breastfed newborn weight loss. J Obstet Gynecol Neonatal Nurs. 2012;41(1):24-32.
doi pubmed - Chantry CJ, Nommsen-Rivers LA, Peerson JM, Cohen RJ, Dewey KG. Excess weight loss in first-born breastfed newborns relates to maternal intrapartum fluid balance. Pediatrics. 2011;127(1):e171-179.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Gynecology and Obstetrics is published by Elmer Press Inc.







